Philips Rethinks Functional Test, Shortening Time to Market
Neil Evans, Senior Manager, Philips
Case Study Highlights
Company—Philips is an $18B international healthcare technology company.
Challenge—Maintain quality despite rising product complexity while reducing cycle time and meeting short development schedules.
Solution—Standardized approach using PXI and LabVIEW to abstract all coding complexity from users without limiting the functionality required to meet stringent test coverage requirements.
Success—Decreased development resources per project by 80%, improved cycle time by 10X, reduced sustaining effort by 10X, and improved test coverage.
Value—Reduced total operating expenses per project by an estimated >$2M.
“The move to a COTS approach using PXI and LabVIEW was critical to this production-test success at Philips. The combination of best-in-class modular hardware along with industry-standard software was pivotal to the millions of dollars and hundreds of hours saved in production test engineering.”
—Neil Evans, Senior Manager, Philips
The Challenge
Philips required a new production-test approach to reduce development schedules and sustaining effort while maintaining or increasing test coverage and quality.
The Solution
Standardized platform was implemented where a common set of code modules and COTS instruments could be configured to meet each test station’s needs.

Ultrasound products are crucial in a variety of applications, including cardiovascular, obstetrics, gynecology, musculoskeletal, liver, and kidney imaging. To lead medical science in these areas, practitioners require higher-resolution images in more accessible form factors. Recent rapid product innovation to meet these demands has created significant and unprecedented time-to-market pressure, which is compounded by growing product complexity, higher production volumes, global manufacturing distribution, increased regulation, and long station-deployment life cycles.
These factors created an executive-level appetite to redesign test approaches, processes, and technology.
- Complexity—Create a platform approach to address the current and future test needs of a diverse portfolio of groundbreaking Ultrasound transducers
- Quality—Significantly improve first pass yield to gain manufacturing efficiency while maintaining high product quality standard
- Life Cycle—Reduce sustaining costs by increasing stability over a 15+ year test-station life cycle
- Development Schedule—Reduce test solution development effort and throughput time per new product introduction by up to 80%
- Manufacturing Volume—
- Increase manufacturing volume of some products by more than 20X, to tens of thousands of units
- Improve manufacturing uptime across a globally distributed supply chain
- Regulation—
- Reduce certification time that can traditionally take up to six months per system
- Reduce post deployment updates that require recertification
- Data—Maintain complete data sets for every test on every DUT
Time-to-market is a key priority at Philips. In order to meet tightening development schedules, a higher-level starting point for new test solutions had to be developed that would allow engineering teams to configure an existing solution to meet their needs rather than start from scratch. Criteria for the standard solution were:
Provide a rapid configuration of test solutions to address new and existing product and subassembly test needs for multiple process steps
Scale to meet future product needs (test-step expansion, signal processing, instrument configurations, and communication/data interfaces) while minimizing additional code development and code recertification
Center on widely available COTS elements to ease global supply-chain and sustaining challenges and significantly reduce the need for custom DUT interfaces
A standardized platform solution was implemented where a common set of code modules and COTS instruments could be configured to meet the test needs of each test station.
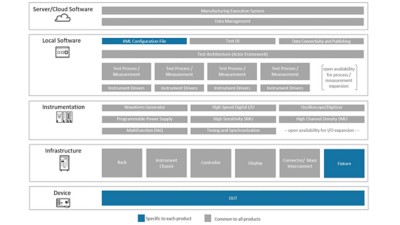
Solution Architecture
We developed a software architecture with minimal dependencies between code modules so that each function can operate in the same fashion, independent of the context within which it runs. This allows for significant code reuse of well-written, verified code, improving time-to-market, code quality, and the number of regulatory recertifications.
When a new product must be tested, the only elements to be developed are the XML configuration file and the fixture. This shortens not only development time, but also certification, as most of the code is unchanged and so existing certification documents can be referenced. This is much like when an orchestra is handed new sheet music—they can play a different tune without changing musicians, instruments, or conductor.
If a product specifies a new process or measurement, the required new code can be written and added to the library without affecting existing framework. It’s similar to when a new orchestral arrangement requires a specialized instrument, such as a grand piano: It can be added without disturbing the existing configuration. Critically, the other musicians can still play all of the other pieces in their repertoire with no changes, while the pianist simply sits in silence. In our case, tests for all previous products used by the platform do not need to be redeveloped or recertified each time we add a new one.
Software Selection
We chose LabVIEW, the industry-standard test engineering tool, for its fully featured hardware-integration capabilities. The LabVIEW Actor Framework is a published software architecture that met this test solution’s extensive needs due to its well-known, tried-and-tested status—we quickly could hire and train contributing engineers for development.
The common proficiency in LabVIEW across Philips provides straightforward path for adoption across the organization of this standard test framework.
Hardware
The primary concern in hardware selection was the measurement capability, precision timing, and stability of the instrumentation. We decided to move from a solution that relied heavily on proprietary hardware to a COTS solution based on PXI because of:
Scalability—Adding new functionality with simple modules keeps time-to-market short.
Coverage—The wide breadth of I/O and firmware functions gives us confidence in complete test coverage, both now and in the future.
Accuracy—PXI has best-in-class measurement accuracy, combined with tight timing/triggering across the chassis backplane, unobtainable with a “rack-and-stack” box instrument approach.
Robustness—PXI’s industry-proven hardware reliability ensures optimum overall equipment effectiveness.
Success
We deployed the system in six globally distributed manufacturing sites and expect significant future adoption.
Results—compared to previous solution testing similar DUTs
- Complexity—Test coverage and signal-path improvements meet the needs of a diverse portfolio of state-of-the-art xMatrix ultrasound transducers
- Quality—Easy access to test data drives significant yield improvements through structured problem-solving
- Life Cycle—
- Significant ongoing cost savings through 10X reduction in resources required to sustain the systems
- Almost no unplanned test downtime exists, thanks to reliable test hardware and verified error-free software
- Absolutely no platform software changes have been required since deployment in 2018
- Development Schedule—We achieved an 80% reduction in NPI test development effort and schedule
- Manufacturing Volume—
- Product-level test cycle time reduced by 10X
- Wafer test cycle time reduced by 5X
- Regulation—Certification validation reduced by four months: From between five and six months to two months
- Data—One global database system delivers complete test traceability
- Executive Sponsorship—The ability to articulate the business value that a test organization could deliver was critical. In this case, we could forecast the exponential development and sustaining costs in-line with increased product complexity. A vision of breaking the relationship between product complexity and test-system cost provided executive buy-in.
- Engineering proficiency—Because any plan is only as good as the people executing it, critical to the creation of this initiative was our recruitment and continual engagement of two leading software architects and one hardware designer who formed our core team. We attribute this project’s success to these lead engineers’ innovation and ongoing diligence as a collaborative team effort.
- Technology Adoption—Moving to a COTS approach using PXI and LabVIEW was critical to our to this production test success at Philips. The combination of best-in-class modular hardware along with industry-standard software was pivotal to the millions of dollars and hundreds of hours saved in production-test engineering.
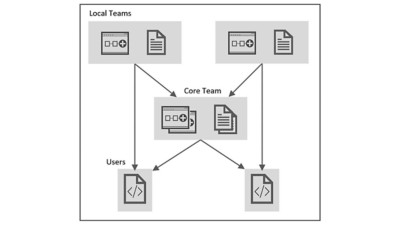
Future Vision
The most significant investment in standardization comes with forming a core team to set up the initial framework. Once complete, adding updates and maintaining code bases is a lighter lift because, at this stage, teams from different organizations can participate as local contributors, leveraging existing test steps and processes while adding to the overall code base when additional functionality is needed. Company-wide, users can utilize an ever-expanding toolset, which they can access through XML configuration files.
The process is governed in the following way:
- A core team develops and curates a general library of functions
- Engineers on different local teams develop libraries of LabVIEW code performing new requirements
- Each code module includes requirements and validation documentation
- Local libraries can be contributed to the general library
- Users create XML files that configure platforms and applications—without the need to understand the underlying LabVIEW code—using a combination of local and general library functions
Further opportunity to optimize product and manufacturing processes exists through better utilizing test data and connected test systems. The large scale of manufacturing creates a data-rich environment, so by harmonizing our data approach across manufacturing sites and products, we can gain greater insights to deliver significant operational benefits.
Authors
Neil Evans, Senior Manager, Product Industrialization Test—Philips Healthcare
Brian Bassett, Senior Product Industrialization Engineer—Philips Healthcare
Davy Hwang, Segment Lead, R&D Innovation—Philips Healthcare
